If you’re in the supplement game, you already know the upside is huge. The industry is booming, the margins are healthy, and people are hungry for products that help them feel better, live longer, and perform at their best.
But there’s a catch…
Marketing dietary supplements in 2025 is like walking a tightrope over shark-infested waters. One wrong move, one poorly worded ad, one overhyped claim and you’re either getting flagged by Meta, delisted on Amazon, or worse, receiving a love letter from the FTC.
I’ve helped hundreds of supplement brands scale to seven and eight figures, and here’s what I can tell you..
You can grow fast
You can build trust
You can market with confidence
…but only if you understand the rules of the game. Let’s break down how to sell supplements without getting smacked by compliance.
First, Know Who You’re Dealing With
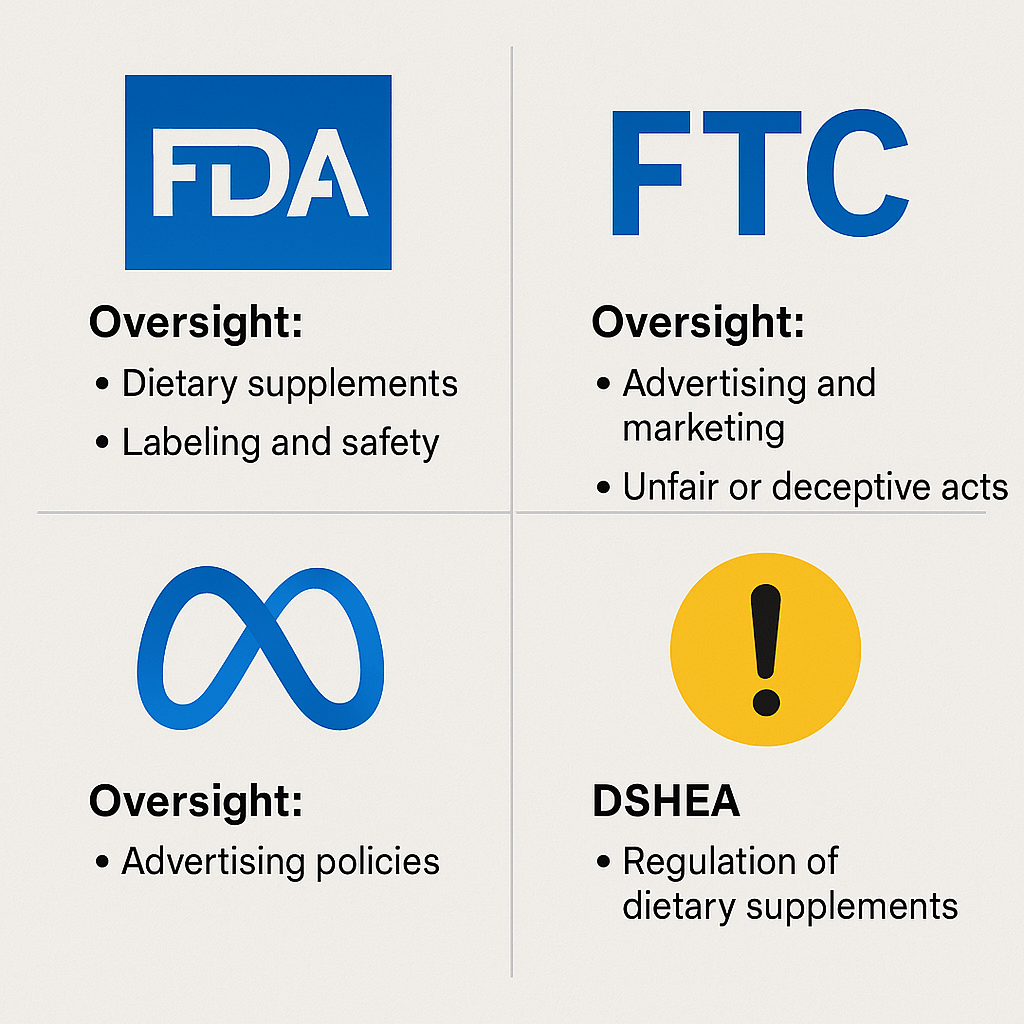
Before we get into tactics, you’ve got to understand who’s actually regulating you, because it’s not just one player.
Here’s the regulatory landscape:
- FDA (Food & Drug Administration): Regulates labeling, manufacturing practices, and ingredient safety. Doesn’t “approve” supplements but will act if you’re making unapproved claims.
- FTC (Federal Trade Commission): Oversees advertising. If you’re making false or misleading claims, the FTC is your problem.
- DSHEA (Dietary Supplement Health and Education Act of 1994): This law outlines how supplements are regulated, separating them from drugs, but still requiring responsible marketing.
- Advertising Platforms (Meta, Google, Amazon): These guys have their own rules, which are often stricter than the government’s.
⚠️ If you’re making a claim that sounds like you’re treating a disease, you’re marketing a drug—not a supplement. That’s the quickest way to get into trouble.
Common Compliance Traps (That Most Marketers Step Right Into)
If you’ve ever had an ad account shut down, a listing removed, or an agency freak out over copy edits, you’re not alone. Here are the top traps that catch supplement brands over and over:
❌ Using Disease Claims
Words like “cures,” “treats,” “prevents,” and “heals” are radioactive. Even if your product does help with something serious like anxiety or diabetes, you can’t say it directly.
❌ Referencing Specific Conditions
This includes everything from arthritis to ADHD to IBS. Even subtle mentions like “reduces inflammation” can be risky without the right context.
❌ Cherry-Picking Clinical Studies
Don’t quote a study about your ingredient curing cancer in rats and imply the same result in humans. You need context, proper citations, and caution.
❌ No FDA Disclaimer
You’ve seen this a thousand times, but if you leave it off your site or marketing materials, you’re asking for problems:
“These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
Put it on product pages, email footers, landing pages—anywhere you’re making claims.
So What CAN You Say?
Here’s where good marketing meets smart compliance. You don’t need to make wild promises to sell effectively, you just need to speak your customer’s language while staying above board.
✅ Use Structure/Function Claims
These are statements about how a product supports normal body functions:
- “Supports healthy blood sugar levels”
- “Promotes joint comfort”
- “Helps maintain a positive mood”
✅ Highlight the Ingredient Story
Instead of saying your product “treats anxiety,” say it includes L-Theanine, a compound shown to support relaxation and calm under stress.
✅ Focus on Lifestyle Benefits
Talk about the outcome your customer wants: better sleep, sharper focus, less fatigue. These are benefits, not medical claims.
Ensure Your Marketing is Compliant


Make Trust Your Competitive Advantage
In a saturated space, trust is the new conversion rate. Consumers are skeptical—and they should be. Half the internet is hawking sketchy powders and capsules.
That’s where you come in with:
- Transparent ingredient sourcing
- Doctor-formulated or expert-backed products
- Third-party testing
- Real reviews and testimonials (carefully worded, of course)
“Compliance isn’t the enemy of creativity, it’s the guardrails that let you scale without crashing.”
Don’t Forget: Platforms Have Their Own Rules
Even if you’re technically compliant with FDA/FTC rules, ad platforms like Facebook, Google, and TikTok might still shut you down for violating their terms.
Meta (Facebook & Instagram)
- Hates “before and after” pics
- Flags “transformational” language
- Rejects ads that imply inadequacy (“Are you tired of being fat?”)
Google Ads
- No disease-related claims
- No misleading headlines
- Landing page must match ad copy and be fully transparent
Amazon
- Very strict about claims in bullet points
- Requires documentation for certain certifications
- Easily flagged by algorithm or automated bots
Final Thoughts
The truth is, you can absolutely grow a powerful, profitable supplement brand without cutting corners. But you need to know the rules and then play to win within them.
Because the brands that win in 2025 aren’t the ones making the biggest promises…
They’re the ones earning the most trust.
Want Help Marketing Your Supplement Brand?
That’s exactly what we do at NutraMarketers. We help supplement brands scale with high-performing, compliant marketing strategies from funnels to ads to email flows and beyond.
FAQ’s
Q: Is it legal to advertise dietary supplements?
Yes, as long as you follow FDA, FTC, and platform-specific rules—especially when it comes to claims.
Q: What is a compliant supplement claim?
Structure/function claims like “supports healthy joints” or “promotes energy” are compliant. Avoid anything that sounds like you’re treating a disease.
Q: Does the FDA approve supplements?
No. The FDA does not approve supplements before they go to market, but it regulates how they’re labeled and marketed after launch.
Q: Do I need a disclaimer on my supplement website?
Yes. Any time you’re making claims about your product, include the FDA’s DSHEA disclaimer.
About the Author

John Smiddy is the founder of NutraMarketers, a full-service marketing agency for supplement brands. He’s the best-selling author of Stop Guessing, Start Marketing and a recognized expert in supplement marketing, ecommerce, and compliance strategy. Over the past decade, he’s helped hundreds of CPG brands scale while staying compliant in a fast-changing market.
